VFDP-14 - Potential Drug Repurposing Scoping
TLDR
We want to explore the feasibility and cost of a drug repurposing trial using an off the shelf compound that has shown promise in the lab autophagy assays. To do this we need to budget to spend up to $30K on IP, regulatory, CRO, & Manufacturing consultants & firms.
Background
Vita-FAST is developing new autophagy-inducing compounds for potential therapeutic applications and systemic cellular longevity. Over the course of the first half of 2025 we identified two novel targets that are activated by our lead series and have previously been proposed as targets for longevity. Further we discovered that there are a group of commercially available compounds that bind to these targets. Testing these commercially available compounds in the lab autophagy assets revealed them to be highly potent inducers of autophagy, the most potent compounds we have ever tested against Viktor’s autophagy assays. We wish to scope the feasibility of taking one of these assets to a longevity clinical trial directly as a repurposing opportunity.
N.b. We are continuing to develop the novel VF compounds as per the experiments detailed in VFDP-13
Objective
To robustly scope the cost and timeline of setting up a Phase 2 clinical trial of a repurposed compound for autophagy induction and longevity. This VFDP is designed to scope if the VITA-FAST project can accelerate rapidly by going directly into humans with a repurposed compound that we have uncovered in the course of our work.
Plan
Thanks to the work of the Molecule team in the UAE, we have excellent connections with the regulator and potential hospitals that have stress tested this idea. Through these conversations with partners, experts, the territory regulator, prospective clinical trial hospital sites and our own work we have identified the key risks as being manufacturing timeline and cost, the regulatory feedback to allow the trial to commence, the feasibility of the clinical trial protocol at the clinical site. Hence we plan to:
-
First file application IP of these compounds repurposed for use in autophagy induction and longevity allowing us to disclose to partners the structures we wish to make and test.
-
Scope the prospective cost and timeline of manufacturing these compounds to GMP standard.
-
Draft the clinical trial protocol for review by the hospital and regulator.
-
File a regulatory pre-submission to gather formal feedback on the plan.
Conducting this scoping in a timely and robust fashion requires engaging with consultants and professionals in each domain and we have identified through either the partners in UAE or our professional network the best experts for each.
Cost Implications
The cost of this is up to $30K as laid out below. Accordingly we seek budget approval for:
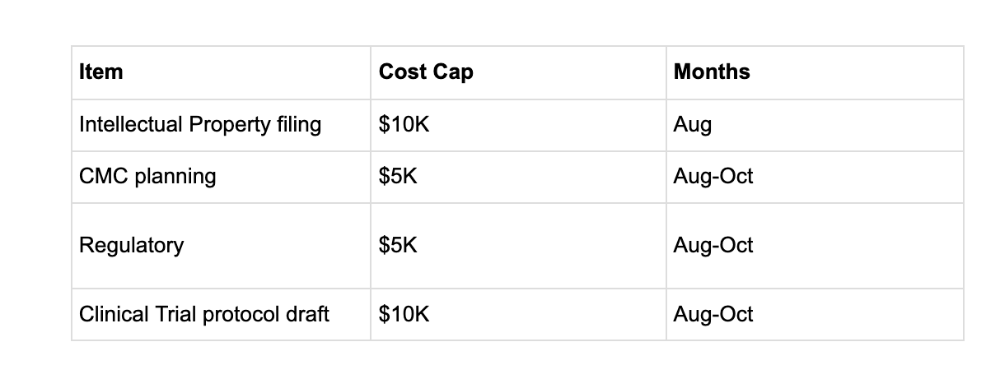
We do not expect that the total cost will reach the full amount, however would like to have budget room for this - we will update this back to the community with final cost numbers from each supplier shortly.
The VITA-FAST project wallet currently has a value of $177,500K (as of 1st Aug 2025) and hence, thanks to fluctuations in the price of $VITA-FAST & of $ETH this spend fits within the existing value of the project wallet without impacting VFDP-13 (the work of which is still underway)
For the purposes of treasury management and to secure resource allocation towards scientific experiments, we also propose to convert 16 ETH to USDC at current market prices.
Recommendation
VITA-FAST has developed novel longevity science - this proposal allows us to scope if we could leapfrog other development steps and be in humans within less than a year. We hence recommend this as a highly efficient way of generating value in the VITA-FAST project for all token holders.
Reporting
Budget scope and update will be given during Q3’25.
Off-Chain Vote
For
14.85K VITA-FAST100%
Against
0 VITA-FAST0%
Abstain
0 VITA-FAST0%
Quorum:114%
Timeline
Sep 03, 2025Proposal created
Sep 04, 2025Proposal vote started
Sep 07, 2025Proposal vote ended
Sep 07, 2025Proposal updated